Using dask with Scanpy#
Warning
🔪 Beware sharp edges! 🔪
dask support in scanpy is new and highly experimental!
Many functions in scanpy do not support dask and may exhibit unexpected behaviour if dask arrays are passed to them. Stick to what’s outlined in this tutorial and you should be fine!
Please report any issues you run into over on the issue tracker.
dask is a popular out-of-core, distributed array processing library that scanpy is beginning to support. Here we walk through a quick tutorial of using dask in a simple analysis task.
This notebook relies on optional dependencies in dask. Install them with:
pip install -U "dask[array,distributed,diagnostics]"
from pathlib import Path
import numpy as np
import dask.distributed as dd
import dask.array as da
import scanpy as sc
import anndata as ad
import h5py
from scipy import sparse
sc.logging.print_header()
scanpy==1.10.0rc2.dev19+ga6126980 anndata==0.10.6 umap==0.5.5 numpy==1.26.3 scipy==1.13.0rc1 pandas==2.2.1 scikit-learn==1.4.0 statsmodels==0.14.1 igraph==0.11.3 pynndescent==0.5.11
Here, we’ll be working with a moderately large dataset of 1.4 million cells taken from: COVID-19 immune features revealed by a large-scale single-cell transcriptome atlas
if not Path("cell_atlas.h5ad").exists():
!wget https://datasets.cellxgene.cziscience.com/82eac9c1-485f-4e21-ab21-8510823d4f6e.h5ad -O "cell_atlas.h5ad"
For more information on using distributed computing via dask, please see their documentation. In short, one needs to define both a cluster and a client to have some degree of control over the compute resources dask will use. It’s very likely you will have to tune the number of workers and amount of memory per worker along with your chunk sizes.
cluster = dd.LocalCluster(n_workers=3)
client = dd.Client(cluster)
Note
In this notebook we will be demonstrating some computations in scanpy that use scipy.sparse classes within each dask chunk. Be aware that this is currently poorly supported by dask, and that if you want to interact with the dask arrays in any way other than though the anndata and scanpy libraries you will likely need to densify each chunk.
All operations in scanpy and anndata that work with sparse chunks also work with dense chunks.
The advantage of using sparse chunks are:
The ability to work with fewer, larger chunks
Accelerated computations per chunk (e.g. don’t need to
sumall those extra zeros)
You can convert from sparse to dense chunks via:
X = X.map_blocks(lambda x: x.toarray(), dtype=X.dtype, meta=np.array([]))
And in reverse:
X = X.map_blocks(sparse.csr_matrix)
Note that you will likely have to work with smaller chunks when doing this, via a rechunking operation. We suggest using a factor of the larger chunk size to achieve the most efficient rechunking.
SPARSE_CHUNK_SIZE = 100_000
DENSE_CHUNK_SIZE = 10_000
Dask provides extensive tooling for monitoring your computation. You can access that via the dashboard started when using any of their distributed clusters.
client
Client
Client-c0396364-eac0-11ee-843b-fa163e42f2a8
| Connection method: Cluster object | Cluster type: distributed.LocalCluster |
| Dashboard: http://127.0.0.1:8787/status |
Cluster Info
LocalCluster
52a3e553
| Dashboard: http://127.0.0.1:8787/status | Workers: 3 |
| Total threads: 18 | Total memory: 62.79 GiB |
| Status: running | Using processes: True |
Scheduler Info
Scheduler
Scheduler-ec5f7bcb-ff41-413b-bdb7-720a04e94bb5
| Comm: tcp://127.0.0.1:36013 | Workers: 3 |
| Dashboard: http://127.0.0.1:8787/status | Total threads: 18 |
| Started: Just now | Total memory: 62.79 GiB |
Workers
Worker: 0
| Comm: tcp://127.0.0.1:43253 | Total threads: 6 |
| Dashboard: http://127.0.0.1:41279/status | Memory: 20.93 GiB |
| Nanny: tcp://127.0.0.1:36245 | |
| Local directory: /mnt/workspace/tmp/dask-scratch-space/worker-2oeq1e55 | |
Worker: 1
| Comm: tcp://127.0.0.1:40683 | Total threads: 6 |
| Dashboard: http://127.0.0.1:45753/status | Memory: 20.93 GiB |
| Nanny: tcp://127.0.0.1:41653 | |
| Local directory: /mnt/workspace/tmp/dask-scratch-space/worker-mnl6y6ds | |
Worker: 2
| Comm: tcp://127.0.0.1:45383 | Total threads: 6 |
| Dashboard: http://127.0.0.1:38031/status | Memory: 20.93 GiB |
| Nanny: tcp://127.0.0.1:34969 | |
| Local directory: /mnt/workspace/tmp/dask-scratch-space/worker-i2wo06l1 | |
We’ll convert the X representation to dask. For more info on i/o from disk, please see the anndata tutorials, e.g. here or here. For now, simply converting X will be enough to demonstrate the functionality of scanpy with dask.
def read_sparse_as_dask(file_pth: str, elem_name: str, stride: int):
with h5py.File(file_pth, "r") as f:
elem = f[elem_name]
shape = elem.attrs["shape"]
if (encoding_type := elem.attrs["encoding-type"]) != "csr_matrix":
raise ValueError(
f"This method was only written for csr_matrix encoding, but a {encoding_type} encoding was found."
)
dtype = elem["data"].dtype
def make_dask_chunk(block_id=None):
# We need to open the file in each task since `dask` cannot share h5py objects when using `dask.distributed`
# https://github.com/scverse/anndata/issues/1105
with h5py.File(file_pth, "r") as f:
mtx = ad.experimental.sparse_dataset(f[elem_name])
(row, _) = block_id
chunk = mtx[
slice(
row * stride,
min((row * stride) + stride, shape[0]),
)
]
return chunk
chunks_0 = (stride,) * (shape[0] // stride)
chunks_0 += (shape[0] % stride,)
chunks_1 = (shape[1],)
da_mtx = da.map_blocks(
make_dask_chunk,
dtype=dtype,
chunks=(chunks_0, chunks_1),
meta=sparse.csr_matrix((0, 0), dtype=np.float32),
)
return da_mtx
The file we’ve retrieved from cellxgene has already been processed. Since this tutorial is demonstrating processing from counts, we’re just going to access the counts matrix and annotations.
%%time
with h5py.File("cell_atlas.h5ad", "r") as f:
adata = ad.AnnData(
obs=ad.experimental.read_elem(f["obs"]),
var=ad.experimental.read_elem(f["var"]),
)
adata.X = read_sparse_as_dask("cell_atlas.h5ad", "raw/X", SPARSE_CHUNK_SIZE)
adata
CPU times: user 2.44 s, sys: 351 ms, total: 2.79 s
Wall time: 2.77 s
AnnData object with n_obs × n_vars = 1462702 × 27714
obs: 'celltype', 'majorType', 'City', 'sampleID', 'donor_id', 'Sample type', 'CoVID-19 severity', 'Sample time', 'Sampling day (Days after symptom onset)', 'BCR single cell sequencing', 'TCR single cell sequencing', 'Outcome', 'Comorbidities', 'COVID-19-related medication and anti-microbials', 'Leukocytes [G over L]', 'Neutrophils [G over L]', 'Lymphocytes [G over L]', 'Unpublished', 'disease_ontology_term_id', 'cell_type_ontology_term_id', 'tissue_ontology_term_id', 'development_stage_ontology_term_id', 'self_reported_ethnicity_ontology_term_id', 'assay_ontology_term_id', 'sex_ontology_term_id', 'is_primary_data', 'organism_ontology_term_id', 'suspension_type', 'tissue_type', 'cell_type', 'assay', 'disease', 'organism', 'sex', 'tissue', 'self_reported_ethnicity', 'development_stage', 'observation_joinid'
var: 'feature_is_filtered', 'feature_name', 'feature_reference', 'feature_biotype', 'feature_length'
We’ve optimized a number of scanpy functions to be completely lazy. That means it will look like nothing is computed when you call an operation on a dask array, but only later when you hit compute.
In some cases it’s currently unavoidable to skip all computation, and these cases will kick off compute for all the delayed operations immediately.
%%time
adata.layers["counts"] = adata.X.copy() # Making sure we keep access to the raw counts
sc.pp.normalize_total(adata, target_sum=1e4)
CPU times: user 9.15 ms, sys: 0 ns, total: 9.15 ms
Wall time: 9.04 ms
%%time
sc.pp.log1p(adata)
CPU times: user 3.81 ms, sys: 0 ns, total: 3.81 ms
Wall time: 3.58 ms
Highly variable genes needs to add entries into obs, which currently does not support lazy column. So computation will occur immediately on call.
%%time
sc.pp.highly_variable_genes(adata)
CPU times: user 1.3 s, sys: 208 ms, total: 1.51 s
Wall time: 43.9 s
PCA currently does not support sparse data. So we will need to densify the expression matrix before passing it in. However, as we are working with only a subset of the data at a time, we are able to perform this operation with a lower memory overhead.
As this is a still a significant increase in memory usage per chunk, we will need to reduce the number of observations present in each chunk.
adata.layers["dense"] = adata.X.rechunk((DENSE_CHUNK_SIZE, -1)).map_blocks(
lambda x: x.toarray(), dtype=adata.X.dtype, meta=np.array([])
)
%%time
sc.pp.pca(adata, layer="dense")
CPU times: user 5.52 s, sys: 588 ms, total: 6.1 s
Wall time: 1min 43s
While most of the PCA computation runs immediately, the last step (computing the observation loadings) is lazy, so must be triggered manually to avoid recomputation.
%%time
adata.obsm["X_pca"] = adata.obsm["X_pca"].compute()
CPU times: user 3.53 s, sys: 924 ms, total: 4.45 s
Wall time: 1min 37s
adata
AnnData object with n_obs × n_vars = 1462702 × 27714
obs: 'celltype', 'majorType', 'City', 'sampleID', 'donor_id', 'Sample type', 'CoVID-19 severity', 'Sample time', 'Sampling day (Days after symptom onset)', 'BCR single cell sequencing', 'TCR single cell sequencing', 'Outcome', 'Comorbidities', 'COVID-19-related medication and anti-microbials', 'Leukocytes [G over L]', 'Neutrophils [G over L]', 'Lymphocytes [G over L]', 'Unpublished', 'disease_ontology_term_id', 'cell_type_ontology_term_id', 'tissue_ontology_term_id', 'development_stage_ontology_term_id', 'self_reported_ethnicity_ontology_term_id', 'assay_ontology_term_id', 'sex_ontology_term_id', 'is_primary_data', 'organism_ontology_term_id', 'suspension_type', 'tissue_type', 'cell_type', 'assay', 'disease', 'organism', 'sex', 'tissue', 'self_reported_ethnicity', 'development_stage', 'observation_joinid'
var: 'feature_is_filtered', 'feature_name', 'feature_reference', 'feature_biotype', 'feature_length', 'highly_variable', 'means', 'dispersions', 'dispersions_norm'
uns: 'log1p', 'hvg', 'pca'
obsm: 'X_pca'
varm: 'PCs'
layers: 'counts', 'dense'
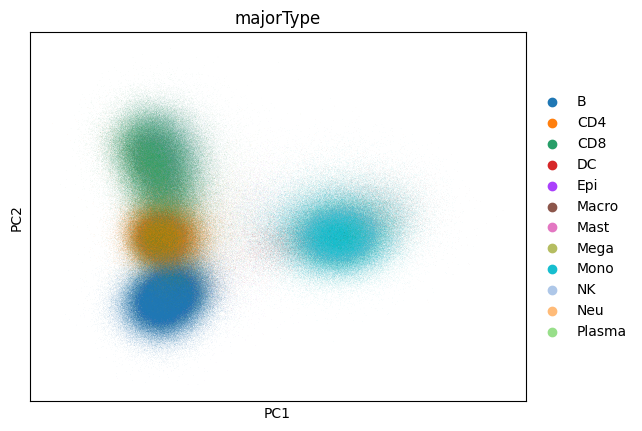
Now that we’ve computed our PCA let’s take a look at it:
sc.pl.pca(adata, color="majorType")
Further support for dask is a work in progress. However, many operations past this point can work with the dimensionality reduction directly in memory. With scanpy 1.10 many of these operations can be accelerated to make working with large datasets significantly easier. For example:
Using alternative KNN backends for faster neighbor calculation Using other kNN libraries in Scanpy
Using the
igraphbackend for clustering
%%time
from sklearn_ann.kneighbors.annoy import AnnoyTransformer # noqa: E402
transformer = AnnoyTransformer(n_neighbors=15)
sc.pp.neighbors(adata, transformer=transformer)
CPU times: user 1min 43s, sys: 2.29 s, total: 1min 46s
Wall time: 1min 23s
%%time
sc.tl.leiden(adata, flavor="igraph", n_iterations=2)
CPU times: user 2min 38s, sys: 4.95 s, total: 2min 43s
Wall time: 2min 42s
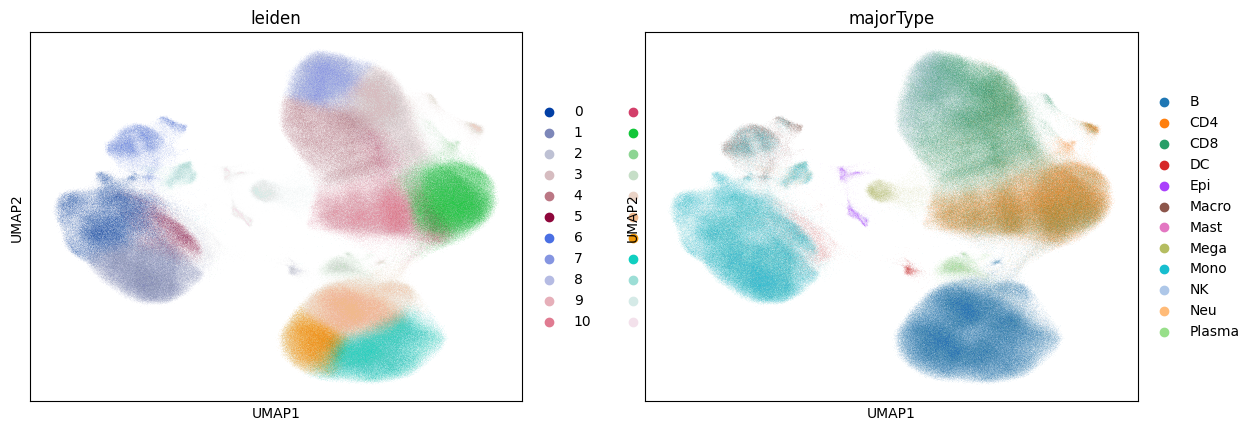
UMAP computation can still be rather slow, taking longer than the rest of this notebook combined:
%%time
sc.tl.umap(adata)
sc.pl.umap(adata, color=["leiden", "majorType"])
CPU times: user 1h 32min 26s, sys: 36min 35s, total: 2h 9min 1s
Wall time: 25min 11s